Do you know how plants take water and minerals from roots? Why does raisin swell up when they are soaked in water? Yes, this is called osmosis.
What is Osmosis?
Osmosis can be defined as a process by which a solvent’s molecules pass from a low concentration solution to a high concentration solution through a semipermeable membrane. It is a passive process and happens without any expenditure of energy. It is also called diffusion of water or solvent.
Endosmosis and exosmosis are two different types of osmosis.
Endosmosis: This is a type of osmosis, which occurs when a substance is placed in a hypotonic solution. In endosmosis, there is a movement of solvent molecules inside or towards the cell, which causes the cell to become turgid or de-plasmolyzed.
For example, when raisins are soaked in water, they swell up after a few minutes. This is because of the presence of higher concentration inside the raisin.
Exosmosis: This is a type of osmosis, which occurs when a substance is placed in a hypertonic solution. In exosmosis, there is a movement of solvent molecules outside or away from the cell and the cell becomes weak or plasmolysed.
For example, when grapes are placed in a highly concentrated sugar solution, they become flaccid.
Osmosis maintains the turgidity of cells and helps plant cells maintain the water level despite the continual water loss due to transpiration.
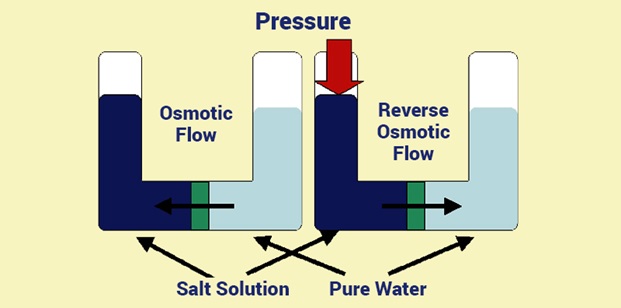
What is Reverse Osmosis?
The movement of molecules against the concentration gradient, within a special dense membrane, is called reverse osmosis. This process is mainly used in the water purification plant to remove salts and other effluents from the water and make it fit for human consumption.
Let’s learn some important information and facts related to osmosis.
Facts About Osmosis
Fact-No-1
In all living systems, osmosis influences water and nutrients’ movement and releases metabolic wastes from the cells.
Fact-No-2
In all plant cells, osmosis is also responsible for absorbing water from the soil and transportation to upper parts of the plant through the xylem.
Fact-No-3
Osmosis is the movement of water through a cell membrane. The pressure that moves water in and out of cells is called water potential.
Fact-No-4
During osmosis, water moves from a region with a high water potential to a region with a lower water potential.
Fact-No-5
The animal cell undergoes lysis when placed in a hypotonic solution, whereas the plant will not burst when placed in a hypotonic solution. This is mainly because the plant cell has dense cell walls, which requires more water.
Fact-No-6
An animal cell exists only in an isotonic solution, whereas plant cells in an isotonic solution are no longer turgid and the leaves of the plant droop. Hence, the hypotonic solution is ideal for a plant cell.
Fact-No-7
Apart from these functions, osmosis also controls sporangia and fruits’ dehiscence and controls the cell to cell diffusion of water.
Fact-No-8
Osmosis plays a vital role in a living organism by maintaining equilibrium inside and outside a cell.
Fact-No-9
Exosmosis occurs when the osmotic pressure is higher, while endosmosis occurs when there is lower osmotic pressure.
Fact-No-10
It stabilizes a living organism’s internal environment by maintaining the balance between intercellular fluid and water levels.
Conclusion
Osmosis is vital and occurs naturally in all biological systems. In this process, solvent molecules move from the region of lower solute concentration into a region of higher solute concentration and are through a selectively permeable membrane.
Here are some common day-to-day examples of osmosis:
- Feeling thirsty after having salty food.
- Swelling of seeds when they are soaked in water.
- Dialysis of the kidney in the human excretory system.
- In animals, the movement of salt-water across our cell membrane.
- In plants- the movement of water, minerals and other nutrients from root nodules to various parts of plants.
These were brief information about some crucial facts about osmosis.
Stay tuned with BYJU’S to learn more about the osmosis, their types, examples and other related topics along with exciting videos by subscribing to BYJU’S YouTube videos.